How to connect copper and aluminum wires
How to connect copper and aluminum wires
Over the years, due to the large amount of aluminum stranded wire and steel core aluminum wire (although copper wire is used now, many old houses and factories still use aluminum wire), a considerable number of copper and aluminum joints will appear between the new and old lines. Since the standard potential of copper and aluminum to hydrogen is very different (aluminum is -1.34 and copper is +0.3448), these two materials are in direct contact, and under certain conditions (such as moisture in the air, carbon dioxide, etc. form an electrolyte), a chemical battery is formed, which produces faster electrochemical corrosion, resulting in an oxide film on the surface of the conductor, increasing the resistance of the joint, and in severe cases, even burning the joint, causing the wire to be broken (according to the company P=I*I*R, the load carried is the same, so the current I is the same. However, because the joint resistance becomes larger and R increases, the power P of this joint will increase at this time, and it will heat and burn out as time increases).
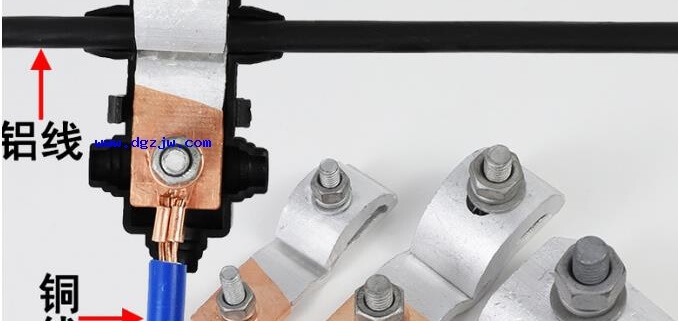
When connecting copper and aluminum wires, in order to avoid electrochemical corrosion, the two should not be directly connected, but should be taken to take copper and aluminum transition measures. That is, use a suitable copper and aluminum transition clamp and connecting pipe to connect the two. In addition, on lines with smaller loads, the copper or aluminum wires can be tinned and then connected can also reduce electrochemical corrosion. This is because the potential difference between copper-tin or aluminum-tin is much smaller than the potential difference between copper-aluminum. There are several common joint transition methods.





Leave a Reply
Want to join the discussion?Feel free to contribute!